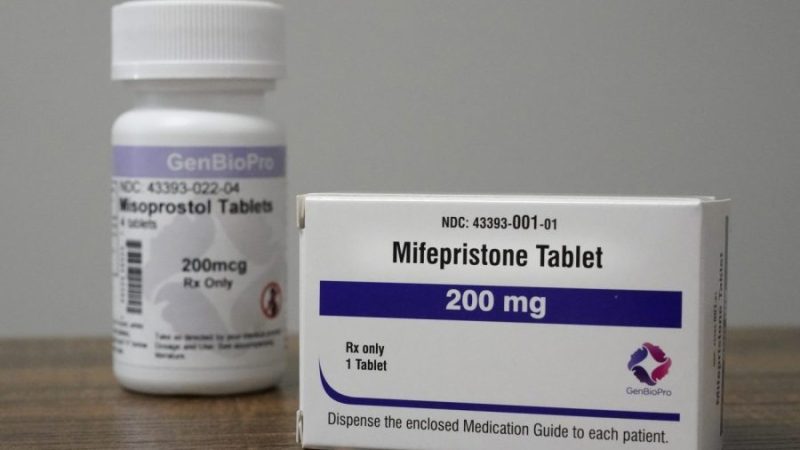
The Department of Justice on Monday said a lawsuit against the Food and Drug Administration seeking to sharply restrict the abortion pill mifepristone should be dismissed, continuing the position of the Biden administration.
In a court filing, the Trump administration argued Idaho, Missouri and Kansas have no ties to Amarillo, Texas, where the lawsuit was filed. The states are free to file in their own districts, the DOJ said.
“Aside from this litigation, the States do not dispute that their claims have no connection to the Northern District of Texas,” the DOJ wrote. “The states cannot keep alive a lawsuit in which the original plaintiffs were held to lack standing, those plaintiffs have now voluntarily dismissed their claims, and the States’ own claims have no connection to this District.”
The states did not file their own lawsuit but instead were granted the ability to intervene in a lawsuit first filed in 2022 by a group of anti-abortion physicians and medical associations.
Last year, the Supreme Court dismissed that lawsuit, saying that private parties had no legal basis to challenge access to mifepristone. The justices found the conservative doctors in the lawsuit did not show they had personally been harmed by the government’s actions regulating mifepristone.
Monday’s filing marks the first time the Trump administration has been asked to weigh in on the case.
The red states claim some of the FDA actions to loosen access to mifepristone allowed the pills to flood across their borders, endangering the lives of women and undermining their anti-abortion laws.
The states are challenging the FDA actions that have loosened restrictions on the drug since 2016, including approving it for use in the first 10 weeks of pregnancy and allowing it to be prescribed by telemedicine and sent through the mail.
The administration also urged dismissal based on timing, arguing the states’ challenge to FDA’s 2016 actions is outside the six-year statute of limitations.
The FDA has repeatedly found that mifepristone is safe and that a medication abortion regimen that includes mifepristone and a second drug, misoprostol, is a safe and effective alternative to surgical abortions.
The Supreme Court’s ruling on the case didn’t address the underlying regulatory or safety issues the plaintiffs raised, instead deciding the case only on standing.